Table of Contents
This is a topic that is often given as a reason by students and residents for choosing a nephrology elective, wanting to understand acid base disorders.
There are multiple ways to assess acid base status.
Nephrologists use the Physiologic approach.
What is this?
- There is an expected “normal” physiologic compensatory response to a primary acid base disorder.
- If the expected compensatory response isn’t present then there is an additional primary acid base disorder, even if the numbers are in the “normal” range. This aids in the identification of mixed acid base disorders.
The Base Excess (or base deficit approach) may seem easy as the base deficit is often reported on blood gas analysis. My opinion is that depending on this approach can lead to an oversimplification. That is, it may tell you if there is a metabolic acidosis, but you may miss mixed disorders.
The Stewart Method is too complicated for me. It talks about things like strong ion difference.
Physiologic Approach to Acid Base Disorders
Figuring out acid base disorders can seem overwhelming. But, like anything else in life, if you have a systematic approach and practice it, it will become routine.
The following is an approach I read about many years ago (can’t remember where) that makes sense to me.
- Do the numbers make sense?
- What is the “emia”?
- What is the “osis”?
- What is the anion gap?
- Is the compensation appropriate?
- What is the gap of the gap (delta-delta)?
Do the numbers make sense?
The pH is dependent on the pCO2 and serum HCO3. If one of these numbers is off then any calculations based on them will be inaccurate. How do you know if the numbers make sense? This can be figured out by the Henderson Hasselbalch equation.
I can tell you the equation, but there’s an app for that.
Plug the HCO3 from the metabolic panel and the pCO2 from the blood gas into the calculator and make sure the pH is close to what the blood gas shows.
Why might the numbers not make sense?
- The blood gas and metabolic panel were done at separate times.
- Venous blood gas used. I typically add .05 to the pH and subtract 5 from the pCO2 for a venous blood gas, although in reality this may only be accurate for central venous blood gasses.
- Excessive anticoagulation (heparinized) blood gas tube
If the numbers don’t make sense stop, repeat the labs and start over.
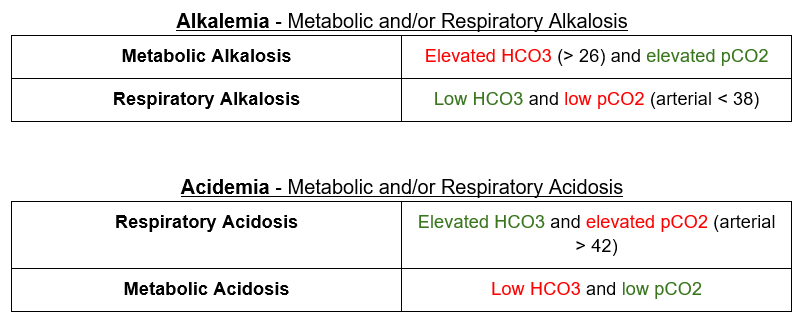
What is the “emia”?
The principle here is that the body doesn’t overcompensate.
- Elevated pH (arterial > 7.42) = alkalemia. If there is an alkalemia there has to be an alkalosis.
- Low pH (arterial < 7.38) = acidemia. If there is an acidemia there has to be an acidosis.
What is the “osis”?
Once we know the “emia” when can figure out the primary “osis”
The primary disorder is highlighted in Red
The compensatory response is highlighted in Green
What is the anion gap?
A normal anion gap is somewhere in the ballpark of 10. If the anion gap is above 20 there is always an increased anion gap metabolic acidosis (no matter what the pH is). The caveats here are:
- There is a range of “normal” for the anion gap. Try to compare it to the patient’s baseline.
- The main unmeasured anion that causes the anion gap is albumin. The lower the albumin, the lower the anion gap. For every 1 g/dl decrease in albumin (less than 4), add 2.5 to get the corrected anion gap.
corrected AG = uncorrected AG + (2.5 × [normal albumin – observed albumin])
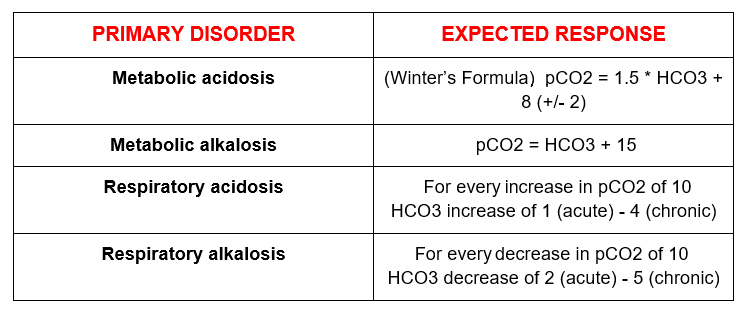
Is the compensation appropriate?
There are formulas to calculate the expected “normal” physiologic response.
These are the ones I use
To become expert at diagnosing mixed acid base disorders you need to understand the following:
Inadequate compensation is a second primary disorder.
Overcompensation (the body doesn’t do this) is a second primary disorder.
What is the gap of the gap (delta-delta)?
Following the above steps will allow you to diagnose a dual mixed acid base disorder (metabolic and respiratory). A triple acid base disorder will have an additional primary disorder. Either:
- A metabolic acidosis and metabolic alkalosis or
- An increased anion gap and normal anion gap acidosis.
The concept is this:
For an increased anion gap acidosis the:
Decrease in HCO3 should = the increase in anion gap
𝝙 Anion Gap (Actual anion gap – 10)
𝝙 HCO3 (24 – actual HCO3)
If it doesn’t, something else is going on. The caveat here is we may not know the patient’s normal anion gap. A reasonable approach is to assume 10, then correct for albumin.
The texts will give you a number of ratios. A ratio of < 1 means 1 thing, a ratio of 1-2 another thing and a ratio of >2 something else. That’s too hard for me to remember. Here’s what I do.
- Take the 𝝙 Anion Gap
- Add it to the actual HCO3

Summary – Why does it matter?
So why bother? It’s fun to try and figure this stuff out, but is it just a mental exercise? The importance is recognizing an underlying clinical condition. By seeing something as an additional primary disturbance (as opposed to under or overcompensation) it allows application of a differential diagnosis. In this way the full clinical picture can be seen.