Table of Contents
The following alcohols can be ingested and lead to toxicity:
- Ethanol
- Ethylene glycol
- Methanol
- Propylene glycol
- Diethylene glycol
- Isopropanol
Although ethanol intoxication may be obvious and levels are readily available, diagnosis of other alcohol toxicity is more difficult. Levels are often send-out tests with results not available in a manner timely enough to make treatment decisions. Given the potential of severe, even fatal toxicity one must have a high clinical suspicion.
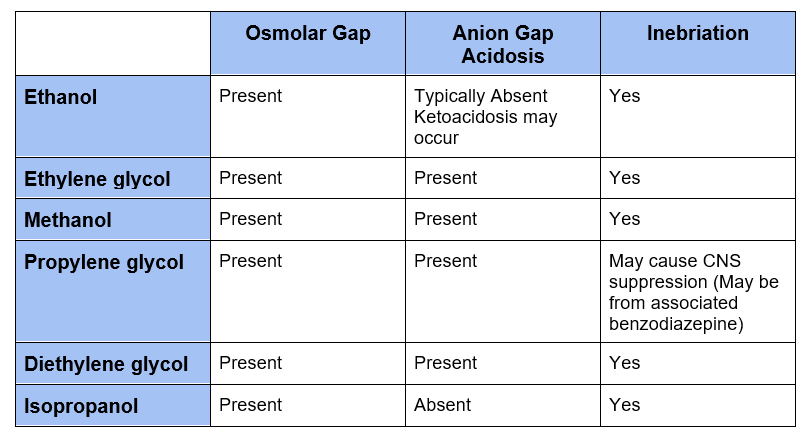
Clinical clues
- Osmolar gap
- Increased anion gap acidosis
- Inebriation
Each of these alcohols is osmotically active, raising the serum osmolarity. An osmolar gap can be a clue to their presence. The parent alcohol is initially metabolized by alcohol dehydrogenase and subsequently by other enzymes (such as aldehyde dehydrogenase). The metabolites are not osmotically active, but often are an organic anion causing an increased anion gap metabolic acidosis in addition to toxicity.
- Accumulation of alcohol ➔ Increase in serum osmolarity (raising osmolar gap)
- Accumulation of metabolite ➔ Increase in anion gap
- Metabolites of ethanol and isopropanol do not cause anion gap
Osmolar gap
All of these alcohols are osmotically active and therefore increase serum osmolarity. A clue to this is the presence of an osmolar gap
What is an osmolar gap? It is the difference between the measured and calculated serum osmolarity.
The main osmotically active substances normally in the blood are:
- Sodium (and it’s associated anion): Measured in Meq/Liter
- Glucose: Measured in mg/dL
- BUN: Measured in mg/dL
Serum osmolarity is measured in mOsm/L so the units must be converted in the calculated anion gap.
- Sodium (and associated anion): No conversion Meq = mOsm
- Glucose:
- Divide by molecular weight (180) to convert mg ➜ mOsm
- Multiply by 10 to convert dL➜ L
- BUN:
- Divide by molecular weight (28) to convert mg ➜ mOsm
- Multiply by 10 to convert dL➜ L
Calculated osmolarity = 2 x Na+ + glucose/18 + BUN / 2.8
As the toxic alcohols may be congested with ethanol and ethanol levels can be readily measured, ethanol can be added to the calculation
- Ethanol: Molecular Weight – 46
- Ethanol/4.6
Calculated osmolarity = 2 x Na+ + glucose/18 + BUN / 2.8 + ethanol/4.6
Normal serum osmolarity is 285-290 mosm/L.
Normal osmolar gap is < 10
Mild increase in osmolar gap (15-20) may be seen in:
- Ketoacidosis
- Lactic acidosis
- Renal failure
- Hyponatremia
An osmolar gap > 20 suggests accumulation of an alcohol
Osmolar gap pitfalls
- When the alcohol is metabolized the osmolar gap will decrease, while the anion gap acidosis may increase.
- Early post ingestion osmolar gap will be more prominent
- Late post ingestion anion gap acidosis will be more prominent.
- The size of the osmolar gap is inversely proportional to the molecular weight of the alcohol.
- Methanol concentration of 50 mg/dl increases the serum osmolarity by @ 15 Mosm/L
- Ethylene glycol concentration of 50 mg/dl increases the scrum osmolarity by @ 9 Mosm/L
- Diethylene glycol has a high molecular weight, so an increased osmolar gap may not be present
Ethanol
- Ketoacidosis uncommon
- Metabolism:
Ethanol ➜ acetaldehyde ➜ acetic acid ➜ acetyl CoA ➜ ketone bodies
- Ketoacidosis when occurs is from deceased insulin and increased lipolysis
Ethylene glycol
- Present in antifreeze
- Ingestion may be accidental or intentional (suicide attempt or in lieu of ethanol)
- Causes inebriation
- Metabolism
Ethylene glycol ➜ glycolaldehyde ➜ glycolic acid ➜ glyoxylic acid and oxalic acid (oxalate)
- Clinical Findings:
- Osmolar gap
- Increased anion gap metabolic acidosis (glycolic acid and oxalic acid)
- Neurologic: Confusion, stupor
- Calcium oxalate crystals on UA (4-8 hours after ingestion)
- Acute Kidney Injury (24-72 hours after ingestion)
- Diagnosis:
- Ethylene glycol levels (sendout)
- Provisional diagnosis based on clinical findings
- Lactate gap
Lactate Gap @BCNephro #Shorts – YouTube
- Clinical Complications:
- Increased anion gap metabolic acidosis
- Acute Kidney Injury (from deposition of calcium oxalate). Occurs after 24-72 hrs
- Cardiac and cerebral damage (from deposition of calcium oxalate)
- Hypocalcemia (from deposition of calcium oxalate)
- High mortality if untreated
- Treatment:
- Fomepizole (inhibits alcohol dehydrogenase blocking conversion to toxic metabolite)
- Ethylene glycol level > 20 mg/dl
- Recent ingestion with osmolar gap > 10 mOsm/L
- Strong clinical suspicion with acidosis pH < 7.3 or HCO3– < 20 mEq/L) and osmolar gap > 20 mOsm/L
- Hemodialysis
- Ethylene glycol level > 50 mg/dl
- Strong clinical suspicion with acidosis pH < 7.25-7.30, or renal failure
- Bicarbonate (if pH < 7.30)
- Thiamine: promotes metabolism of glycolic acid
- Pyridoxine: promotes metabolism of glyoxylate
- Volume expansion
- Fomepizole (inhibits alcohol dehydrogenase blocking conversion to toxic metabolite)
Methanol
- Present windshield wiper fluid, antifreeze, model airplane fuel.
- Ingestion may be accidental or intentional (suicide attempt or in lieu of ethanol)
- Cause inebriation
- Metabolism
Methanol ➜ formaldehyde ➜ formic acid (formaldehyde dehydrogenase) ➜ CO2 and H20
- Clinical findings:
- Osmolar gap
- Increased anion gap metabolic acidosis (formic acid)
- Visual symptoms/findings
- Decreased acuity, photophobia, blurred vision
- Decreased pupillary light reflex, hyperemia of optic disk
- Abdominal pain
- Neurologic symptoms
- Confusion, stupor, coma
- Clinical Complications:
- Increased anion gap metabolic acidosis
- Neurologic: basal ganglia damage (rigidity, tremor, masked facies)
- Blindness
- High mortality if untreated
- Diagnosis:
- Methanol levels (often sendout)
- Provisional diagnosis based on clinical findings
- Treatment:
- Fomepizole (inhibits alcohol dehydrogenase blocking conversion to toxic metabolite)
- Methanol level > 20 mg/dl
- Recent ingestion with osmolar gap > 10 mOsm/L
- Strong clinical suspicion with acidosis pH < 7.3 or HCO3– < 20 mEq/L) and osmolar gap > 20 mOsm/L
- Hemodialysis:
- Methanol level > 50 mg/dl
- Strong clinical suspicion with acidosis pH < 7.25-7.30, visual abnormalities, or renal failure
- Bicarbonate (if pH < 7.30)
- Folate (to enhance metabolism of formic acid)
- Fomepizole (inhibits alcohol dehydrogenase blocking conversion to toxic metabolite)
Propylene glycol
- Vehicle in medication (IV lorazepam, diazepam)
- Toxicity can occur with prolonged high dose infusions (levels > 100 mg/dl)
- Metabolism:
Propylene glycol ➜ lactaldehyde ➜ lactate
- Clinical Findings:
- Osmolar gap
- Increased anion gap metabolic acidosis (lactic acid)
- Acute Kidney Injury
- Clinical Complications:
- Increased anion gap metabolic acidosis (lactic acid)
- Acute Kidney Injury
- Low mortality
- Diagnosis:
- Propylene glycol levels (sendout)
- Provisional diagnosis based on clinical findings
- Treatment:
- Discontinuation of medication
- Hemodialysis (rarely required)
Isopropanol (Isopropyl alcohol)
- Present in rubbing alcohol, hand sanitizer
- Ingestion may be accidental or intentional (suicide attempt or in lieu of ethanol)
- Causes inebriation
- Metabolism:
- Isopropanol ➜ acetone
- Clinical findings:
- Osmolar gap
- Not associated with acidosis (metabolite is acetone)
- GI: Abdominal pain, nausea, vomiting, diarrhea
- Neurologic: Altered mentation
- Positive nitroprusside reaction (ketone test strip)
- Diagnosis:
- Isopropanol level (sendout)
- Provisional diagnosis based on clinical findings
- Clinical complications:
- Hypotension with coma (rare)
- Treatment:
- Supportive
- Hemodialysis (if hypotension, coma)
Diethylene glycol
- Present in contaminated medications or commercial products (i.e. brake fluid)
- Outbreaks occur when used as solvent in medications (illegally). Children often affected.
- Metabolism:
Diethylene glycol ➜ 2-hydroxyethoxyacetaldehyde ➜ 2-hydroxyethoxyacetic acid
- Clinical Findings:
- Osmolar gap less frequent (high molecular weight)
- Increased anion gap metabolic acidosis (2-hydroxyethoxyacetic acid)
- GI: Abdominal pain, diarrhea, hepatitis, pancreatitis
- Neurologic: Altered mental status
- Acute Kidney Injury
- Diagnosis:
- Diethylene glycol levels (sendout)
- Provisional diagnosis based on clinical findings
- Clinical Complications:
- Acute Kidney Injury
- Metabolic acidosis
- Neurologic: Cranial nerve palsies
- Associated with high mortality
- Treatment:
- Fomepizole (inhibits alcohol dehydrogenase blocking conversion to toxic metabolite)
- Hemodialysis
Summary
Toxic alcohol ingestions may be associated with severe, fatal complications. The presence of both an increased osmolar gap and anion gap acidosis suggests their presence. However, as the osmolar gap is from the parent alcohol and the anion gap is from the metabolite they may not be present concomitantly. This makes a high clinical suspicion necessary.