Table of Contents
Which patients with kidney disease should be screened for monoclonal gammopathy?
Assessment for paraproteins is important for the evaluation of most kidney diseases.
Paraproteins can be associated with a wide spectrum of renal manifestations including:
- Acute Kidney Injury
- Chronic Kidney Disease
- Proteinuria +/- Nephrotic syndrome
- Proximal Tubulopathies (i.e. Fanconi syndrome)
Patients should be screened if they have unexplained:
- Acute Kidney Injury (especially if associated with hypercalcemia)
- Chronic Kidney Disease (especially if associated with proteinuria)
- Proteinuria
- Overt albuminuria
- Non albumin proteinuria
- Proximal tubular dysfunction (glucosuria, hypophosphatemia)
What kidney diseases are associated with monoclonal gammopathy?
It is widely known that Multiple Myeloma can cause many renal manifestations.
7 Ways Myeloma affects the Kidney @BCNephro – YouTube
B Cell lymphoproliferative disorders can also cause these same manifestations
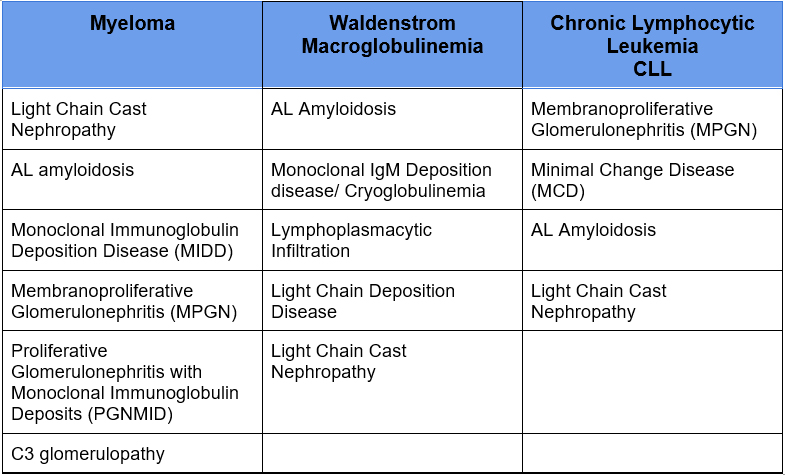
The monoclonal protein can also cause these renal manifestations even if it is not malignant or pre malignant. That is, the monoclonal paraprotein does not meet criteria for myeloma and there is no evidence of lymphoma
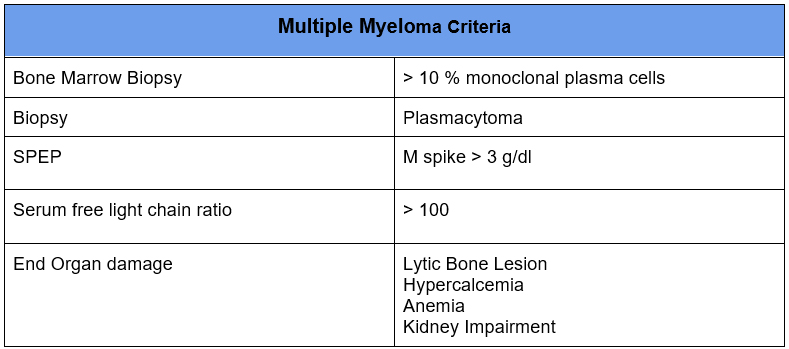
Monoclonal paraproteins that don’t meet criteria for multiple myeloma historically have been referred to as Monoclonal Gammopathy of Undetermined Significance (MGUS).
When the monoclonal paraprotein causes kidney disease in the absence of myeloma or lymphoma it is referred to as Monoclonal Gammopathy of Renal Significance (MGRS). These tend to be progressive with a high incidence of progression to ESRD and recurrence in a renal transplant.
How Do Monoclonal Proteins Cause Kidney Disease?
Light chains are normally filtered by the glomerulus and reabsorbed by the proximal tubule. The normal light chain excretion is < 30 mg/ day.
When these light chains are produced in excess they overwhelm the proximal tubular absorption and result in what is referred to as overflow proteinuria.
However, it is not only the amount of the monoclonal light chain that predisposes to kidney damage. The physicochemical characteristics of the specific clone are important.
Mechanisms of kidney damage. Toxic light chains may:
- More likely to aggregate
- Have an affinity to bind to uromodulin (Tamm Horsfall protein) predisposing to intratubular cast formation
- Cause direct tubular toxicity. Reabsorption of large amounts of light chains may cause proximal tubular damage
Evaluations of Patients with Kidney Disease for Monoclonal Gammopathy
Initial evaluation
Most patients with unexplained AKI, CKD, or proteinuria kidney disease should be screened for a monoclonal gammopathy.
My initial testing includes:
- Serum free light chain (FLC) ratio
- Urinalysis
- Urine protein creatinine ratio
- Urine albumin creatinine ratio
Interpreting Serum FLC ratio
Serum free light chains are normally filtered by the glomerulus. With a decrease in GFR the serum levels of both kappa and lambda light chains will increase.
Since we are looking for a monoclonal protein we are looking for an increased ratio of kappa to lambda (or lambda to kappa) light chains.
If both light chains are increased with a normal ratio, this is not significant.
Kappa light chains are more influenced by a decrease in GFR than lambda light chains. The kappa lambda ratio is often mildly increased with CKD.
- GFR > 60: Normal serum FLC ratio < 1.65
- GFR < 60: Normal serum FLC ratio < 3.10
Interpreting Urinary Studies
- The majority of proteinuria in glomerular diseases is albumin.
Proteinuria: What is it and How I Treat | BCNephro
- In glomerular diseases albumin with be approximately 55-60% of the total protein
- If the urine albumin creatinine to urine protein creatinine is much less than this, < 40% this suggests non albumin proteinuria. This raises my suspicion for light chain proteinuria
- Glomerular proteinuria (albuminuria) can also be seen in paraprotein related kidney diseases such as amyloidosis.
https://bcnephro.com/amyloidosis/
Follow up of Abnormal Screening Studies
There is a dilemma. Both Monoclonal Gammopathy (MGUS) and Chronic Kidney Disease (CKD) are common with increased incidences with aging.
- 3-4% of people over 50 have a monoclonal protein.
- Studies have shown 37-44% of patients with a monoclonal gammopathy also had evidence of CKD.
Therefore a significant number of patients will have both MGUS and CKD as opposed to a MGRS causing the kidney disease.
Although kidney disease and anemia can be considered evidence of end organ damage in myeloma, the kidney disease may be coincident and not caused by the monoclonal protein and the anemia may be caused by the CKD.
So, How do we determine which patients have concomitant MGUS and CKD and which have MGRS causing the kidney disease.
There are a number of additional follow up studies that can be performed. These are an attempt to determine if active myeloma is present. These include:
- SPEP and serum immunofixation
- UPEP and urine immunofixation
- Skeletal survey for lytic bone lesions
- Bone marrow biopsy
- Extra renal biopsy (i.e. fat pad for amyloidosis)
Renal Biopsy
The definitive way to determine if the monoclonal gammopathy is a kidney biopsy. However, which patients should have a kidney biopsy? This can be a difficult clinical question.
This study provides some insight. It reviewed the kidney biopsy results in patients with monoclonal gammopathies.
Here’s what it found:
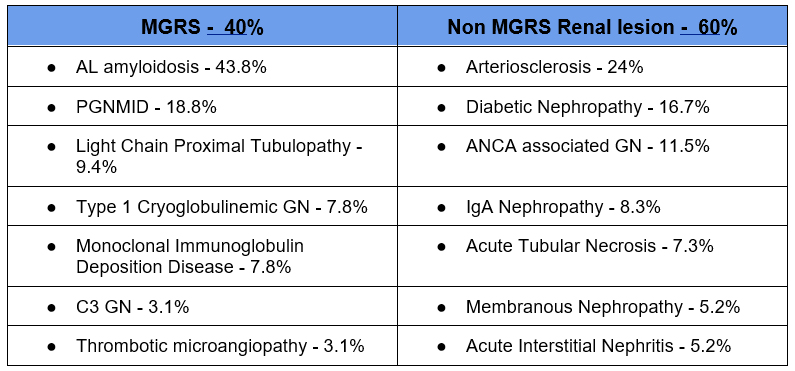
Predictive factors of finding a MGRS lesion on Renal Biopsy
- Proteinuria > 1.5 g/day
- Sensitivity 67.2
- Specificity 58.5%
- Hematuria
- Elevated affected/unaffected serum free light chain ratio:
- MGRS: FLC ratio 5.64 (2.63-21.26)
- Non MGRS: FLC ratio 1.79 (1.06-3.50)
- Lower C3
- Abnormal Bone marrow biopsy
- MGRS: 89.8%
- Non MGRS 7%
Diabetes decreased odds:
https://bcnephro.com/diabetic-nephropathy/
- 20% of diabetics had MGRS lesion on biopsy (most likely amyloidosis)
- Higher proteinuria increased odds for MGRS lesion in diabetics
Summary
Monoclonal gammopathies are an important cause of a wide range of kidney diseases. For this reason it is important to screen for these in unexplained AKI, CKD, or proteinuric kidney diseases. Given the relatively high prevalence of MGUS many patients will have an abnormal screen such as an elevated serum FLC ratio. Renal biopsy is necessary to distinguish MGUS from MGRS. Predictors of an MGRS lesion on renal biopsy include proteinuria > 1.5 grams per day, hematuria, and a more significant elevation in serum FLC ratio (> 3-5).