Table of Contents
- 1 Increased Anion Gap Metabolic Acidosis
- 2 What Causes an Increased Anion Gap Metabolic Acidosis
- 3 What else can you do to help determine the cause of an increased anion gap Metabolic Acidosis?
- 4 What Causes a Normal Anion Gap Metabolic Acidosis.
- 5 What else can you do to diagnose a normal anion gap Metabolic Acidosis?
- 6 Summary
You’ve evaluated an acid base disorder.
You diagnosed a metabolic acidosis:
- pH: Low
- Serum Bicarbonate (HCO3): Low
- pC02: Low
How do you figure out what caused it? This article will provide a framework for differential diagnosis – looking at the:
- Anion Gap – Is it elevated?
- Delta – Delta – What’s that?
- Osmolar Gap
Increased Anion Gap Metabolic Acidosis
First of all – what is the anion gap?
In your body the number of cations = the number of anions (you’re not electrically charged). We don’t measure all of the anions and all of the cations. By convention when calculating the anion gap we measure Sodium, Chloride and Bicarbonate. There are other unmeasured anions (albumin; phosphates, sulfates, small organic acids) and cations (calcium, magnesium, gamma globulins)
So the anion gap = Unmeasured anions – Unmeasured cations
If the anion gap is increased it suggests another unmeasured anion is present (such as lactate or beta-hydroxybutyrate)
Things to remember:
- There is a wide reference range for a normal anion gap. Although we think of a normal anion gap being 8-12, depending on the specific lab the normal range may be as low as 4 and as high as 16. When determining if it is “increased” or “normal” it is most useful to compare it to the patient’s baseline.
- Corrected anion gap. The main unmeasured anion that contributes to the anion gap is albumin. Don’t be fooled by an anion gap that appears “normal” in a hypoalbuminemic patient.
Corrected anion gap – for every 1 g/dl decrease in albumin, add 2.5 to the anion gap
- If the anion gap is > 20, an increased anion gap acidosis is present (regardless of the pH or serum bicarbonate)
What Causes an Increased Anion Gap Metabolic Acidosis
Let’s figure out what the unmeasured anion is that’s causing the acidosis. We all know about lactic acid and diabetic ketoacidosis (DKA), but there’s other things to know.
MUDPILES
There are mnemonics to assist in remembering the differential diagnosis. The one I learned in medical school was MUDPILES (I’m old).
M – Methanol
U – Uremia
D – Diabetic Ketoacidosis (DKA)
P – Paraldehyde
I – Iron, INH
L – Lactic Acidosis
E – Ethylene glycol
S – Salicylates
This is outdated. Paraldehyde?, INH? Really? Who has seen a metabolic acidosis caused by those things? It also misses some important causes of increased anion gap acidosis that we do see.
GOLDMARK
So, someone came up with a new updated (and in my opinion better) mnemonic GOLDMARK. This was 2008 so not that new anymore.
GOLD MARK: an anion gap mnemonic for the 21st century – The Lancet
G – Glycols – ethylene glycol and propylene glycol
O – Oxoproline
L – L- lactate
D – D- lactate
M – Methanol
A – Aspirin
R – Renal Failure
K – Ketoacidosis (Diabetic, starvation, alcoholic)

Tip: Isopropyl alcohol (rubbing alcohol) will result in an elevated osmolar gap with ketosis (but does not cause an acidosis).
What else can you do to help determine the cause of an increased anion gap Metabolic Acidosis?
Osmolar Gap
This is where you try to find out if there is an unmeasured osmole present. Unmeasured osmoles include:
- Ethanol
- Ethylene glycol
- Methanol
- Propylene glycol
Ethanol doesn’t cause a metabolic acidosis, but the other 3 do
Osmolar gap = Measured osmolarity – calculated osmolarity
Calculated osmolarity = ( 2 x Na + glucose/18 + BUN/2.8)
A normal osmolar gap is < 10. If it is elevated in the right clinical context (such as a patient with an increased anion gap metabolic acidosis) you need to think about ethylene glycol or methanol toxicity..
Delta – Delta
This is a way to try to figure out if there’s only an increased anion gap metabolic acidosis or if there’s something else going on too (a metabolic alkalosis or an additional normal anion gap metabolic acidosis).
Delta anion gap – Delta HCO3
[Patient’s Anion gap – normal anion gap (10)] – [Normal HCO3 (24) – patient’s HCO3}
Pitfall:
- Assumption that the normal anion gap is 10. This isn’t necessarily true for every patient. It is better if you know the patient’s baseline anion gap.
Interpretation:
- Ratio of 1 suggests ketoacidosis (that’s because you pee out some of the ketoacids)
- Ratio of 1-1.6 suggests lactic acidosis
That’s too much for me to remember. So what I do is this:
- Add the change in anion gap to the patient’s HCO3. If that number is:
- Low ( <22) – suggests an additional normal anion gap acidosis
- High (>28) – suggests a concomitant metabolic alkalosis.
What Causes a Normal Anion Gap Metabolic Acidosis.
Also referred to as hyperchloremic metabolic acidosis. If the bicarbonate is low and the anion gap is normal the chloride must be high
There’s a mnemonic for that too: HARDUP – is the one I learned.
HARDUP
H – Hyperalimentation
A – Acetazolamide
R – Renal Tubular Acidosis
D – Diarrhea
U – Ureteral sigmoid fistula
P – Pancreatic Fistula
There’s another one too: HARD-ASS
HARD-ASS
H – Hyperalimentation
A – Acetazolamide
R – Renal Tubular Acidosis
D – Diarrhea
A – Addison’s Disease
S – Spironolactone
S – Saline Infusion
The vast majority of cases will be from diarrhea or saline infusion. Medications and RTA are less frequent.
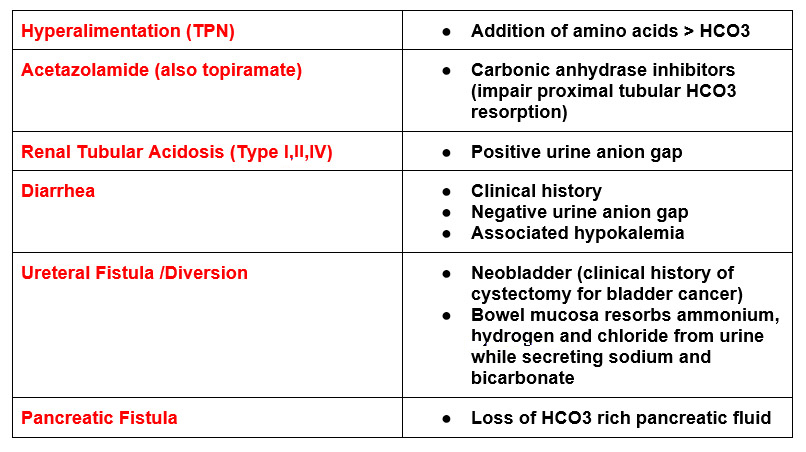
Tip: One of the most common causes of acute hyperchloremic metabolic acidosis is fluid resuscitation with isotonic saline (NS).
What else can you do to diagnose a normal anion gap Metabolic Acidosis?
- Urine Anion Gap
- Urine Osmolar Gap
The way the kidneys excrete the majority of acid is in the form of Ammonium (NH4+). If the kidneys are excreting ammonium in the setting of an acidosis then they are responding appropriately and the acidosis is from an extrarenal source (such as diarrhea). If there’s an acidosis and the kidneys aren’t excreting ammonium then they are not responding appropriately (such as a renal tubular acidosis).
Urine NH4+ cannot be routinely measured in the urine, so we deduce its presence by the urine anion gap. Since NH4+ is positively charged it needs to bring something negatively charged with it.
Urine Anion Gap
(Urine Sodium + Urine Potassium) – Urine Chloride
If this is negative then there must be something positive that you’re not measuring in the urine (such as NH4+). If it’s negative there isn’t much NH4+ present.
The urine anion gap is not reliable if the urine pH is > 6.5. This is because a high urine pH suggests the presence of another anion (besides chloride), such as bicarbonate.
Urine Osmolar Gap
Measured Urine Osmolarity – Calculated Urine Osmolarity
Calculated Urine Osmolarity = (2 x Na + 2 x K + urea + glucose)
(convert urea and glucose from mg/dl to mmol/L)
This is another way to deduce if ammonium is in the urine, by determining that there is something present that is not measured.. If the urine osmolar gap is > 100, it suggests there is ammonium present indicating an appropriate renal response to the acidosis.
Summary
The evaluation of metabolic acidosis hinges on determining if the anion gap is elevated or normal. Remember to correct the anion gap if hypoalbuminemia is present. Clinical history is key. Further evaluation for increased anion gap acidosis includes osmolar gap and delta – delta and for normal anion gap acidosis includes urine anion gap.