Table of Contents
Immunosuppression is required for kidney transplantation. The foreign antigens on the kidney allograft are recognized by the patient’s immune system. Without immunosuppression this will lead to rejection of the kidney.
Immunosuppression is composed of induction and maintenance. The immunologic risk of rejection is highest immediately after transplant and decreases with time. Most cases of acute rejection occur within the first 6-12 months post transplant. Although the intensity of immunosuppression may be decreased over time, in almost all cases indefinite immunosuppression is required.
This immunosuppression is targeted against lymphocytes.
- T lymphocytes: mediate cellular rejection
- B lymphocytes: mediate humoral rejection
Induction Immunosuppression
In addition to the chronic immunosuppression as outlined below most kidney transplant recipients will receive additional induction therapy.
Induction therapy is typically either:
T cell depleting antibodies
- Thymoglobulin – rabbit antithymocyte globulin. This is a polyclonal antibody created in rabbits against human thymocytes. Most often used.
- Alemtuzumab – monoclonal (anti-CD52 antibody). This targets both B and T lymphocytes (rarely used).
Interleukin 2 (IL-2) receptor blocking antibodies
- IL-2 induces T cell proliferation
- Basiliximab is an antibody against CD25, which is the alpha chain of the IL-2 receptor on T lymphocytes. Therefore it blocks the effects of circulating IL-2.
Choice of induction therapy
- Very low immunologic risk (i.e. HLA matched living related donor): No induction
- Low immunologic risk: Basiliximab or Thymoglobulin
- High immunologic risk: Thymoglobulin
Thymoglobulin is more effective in preventing rejection in kidney transplant recipients with high immunologic risk.
Maintenance Immunosuppression
In almost all cases indefinite immunosuppression is required. Most often a three drug regimen is required, although sometimes a two drug regimen may be sufficient. It is extremely rare for a kidney transplant recipient to be only on a single immunosuppressive agent.
Maintenance immunosuppressive agents used for renal transplant include:
- Calcineurin inhibitors
- Tacrolimus
- Cyclosporine
- Antimetabolites
- Mycophenolate
- Azathioprine
- Glucocorticoids
- Most often prednisone
- Costimulatory blockers
- Belatacept
- Mammalian target of rapamycin (mTOR) inhibitors
- Sirolimus
- Everolimus
Immunosuppression Regimens
Most Common: 3 drug regimen
- Calcineurin inhibitor. Most often tacrolimus
- Most often mycophenolate
- Most often prednisone
Steroid Free: 2 drug regimen
- Calcineurin inhibitor. Most often tacrolimus
- Most often mycophenolate
Alternate regimens: Typically used only if there is a condition or complication that precludes the use of one of the standard immunosuppressive regimens.
- Calcineurin inhibitor and glucocorticoid (if contraindication to mycophenolate)
- Belatacept, antimetabolite and glucocorticoid (if contraindication to calcineurin inhibitor)
- mTOR inhibitor and calcineurin inhibitor (tacrolimus) +/- glucocorticoid (if contraindication to mycophenolate). Typically NOT used with cyclosporine
- mTor inhibitor and antimetabolite +/- glucocorticoid (if contraindication to calcineurin inhibitor)
Calcineurin inhibitors
Calcineurin inhibitors are the mainstay of immunosuppression for renal transplant.
Calcineurin stimulates the production of IL-2 mRNA. Calcineurin inhibitors will inhibit this process. There are 2 calcineurin inhibitors: Cyclosporine and Tacrolimus
Cyclosporine
- Was the initial calcineurin inhibitor.
- Two formulations:
- Non modified (Sandimmune)
- Modified-microemulsion (Neoral, Gengraf)
- The modified microemulsion has better and more predictable absorption. The non modified formulation is not typically used
- Currently used mainly in transplant recipients of high vintage (who had been on cyclosporine since the time of their transplant) or in patients with an intolerance to tacrolimus.
Tacrolimus
- Almost universally the calcineurin inhibitor of choice.
- Considered a better immunosuppressive than cyclosporine
- Formulations:
- Short acting. Capsules or liquid formulation. Contents of the capsule can also be administered sublingually. Dosed every 12 hours
- Long acting. Dosed every 24 hours
- Envarsus XR – extended release tablets
- Astagraf XL – extended release capsules
Envarsus is used more often than Astagraf. When converting from short acting tacrolimus to Envarsus (but not Astagraf) there is a dose reduction of 20-30%
- IV tacrolimus. ⅕ – ⅓ daily dose as continuous IV infusion.
- Not often used.
- Associated with risk of anaphylaxis, nephrotoxicity, and neurotoxicity
- Strongly recommended to continue enterally (i.e liquid via nasogastric tube) or administer contents of capsule sublingually
- Some (but not all) recommend a 50% dose reduction from oral to sublingual tacrolimus
As calcineurin inhibitors are the most important immunosuppressive agents they should almost never be held, the exception being if there are supratherapeutic toxic levels.
Monitor AM trough levels.
- Typical target tacrolimus maintenance trough is 5-7, although some use 4-6.
- If trough out of target range
- The 1st thing is to verify that it was a trough, often a problem in the hospital, either the medication was given before lab draw, or late in the evening and morning labs drawn early.
- The 2nd thing to investigate is if there are other medications that affect levels.
- I am reluctant to change doses in hospital as I think more often than not it is a timing issue. Reserve changes for if there is an interfering medication or evidence of toxicity.
Medications that affect Calcineurin Inhibitor Levels
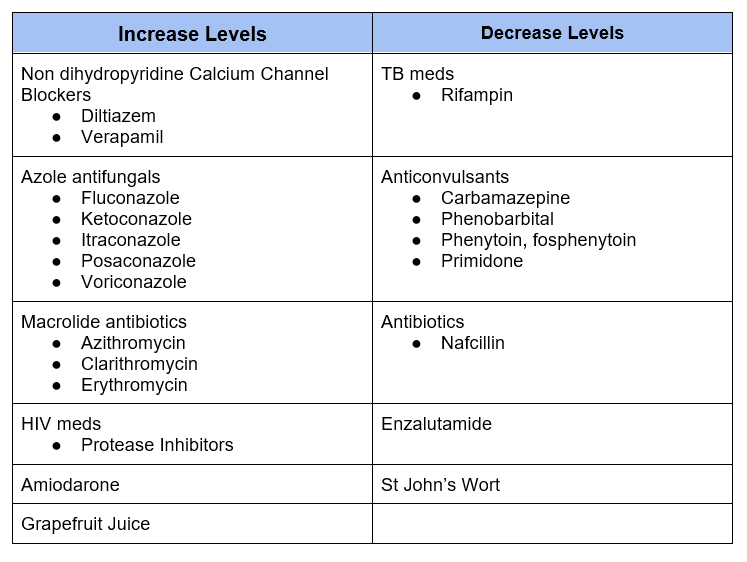
Dihydropyridine calcium channel blockers (ie amlodipine, nifedipine) are considered okay to use with calcineurin inhibitors.
Proton pump inhibitors (PPI’s) may affect levels of calcineurin inhibitors, but can be used. Patients should be advised to have levels checked if PPI started or discontinued and to take PPI consistently (as opposed to intermittent as needed dosing).
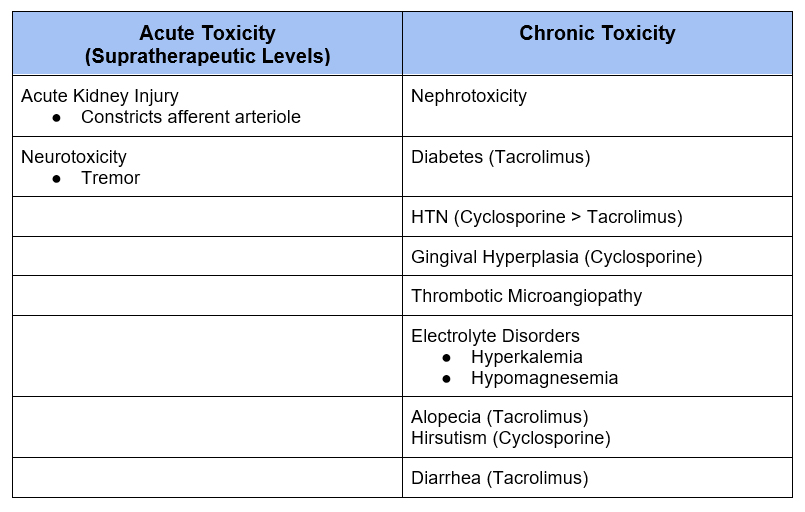
In addition to infection risk from immunosuppression, calcineurin inhibitors are associated with a number of side effects.
Antimetabolite
Antimetabolites Interfere with (nucleotide synthesis) synthesis of nucleic acids impairing production of B and T lymphocytes.
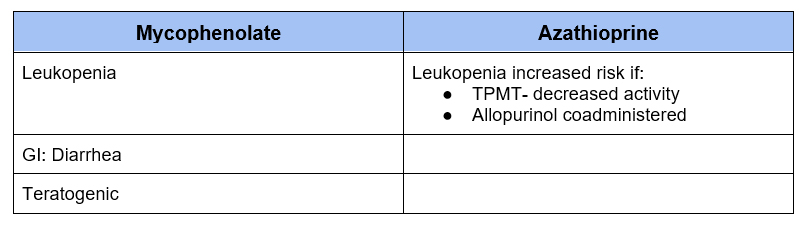
Azathioprine
- The initial antimetabolite has largely been replaced by Mycophenolate
- May be used if intolerance to Mycophenolate or planned pregnancy (as mycophenolate is teratogenic)
- Do not use with allopurinol as combination may result in severe leukopenia
Mycophenolate
- Most often used as part of both the 3 drug (with calcineurin inhibitor and glucocorticoids) and 2 drug (steroid free) regimens
- Two formulations
- Mycophenolate Mofetil (Cellcept)
- Enteric coated Mycophenolic acid (Myfortic) – associated with less GI side effects
- 250 mg Mycophenolate Mofetil = 180 mg enteric coated Mycophenolic acid
The antimetabolite can be held in certain situations.
- Acutely – If severe infection or leukopenia
- Chronically – If need to decrease immunosuppression (frailty, malignancy) or severe adverse effects ( leukopenia, diarrhea)
- If the antimetabolite is held at least 2 other immunosuppressive medications need to be given (typically a calcineurin inhibitor and steroids). If the patient was on a steroid free regimen, a glucocorticoid should be added.
Adverse Effects of Antimetabolites
Glucocorticoids
Glucocorticoids are anti-inflammatory agents that affect the immune system in numerous ways.
In 3 drug regimens prednisone is often used. Initial higher doses are tapered to a typical maintenance dose of 5 mg a day.
Glucocorticoids are associated with many adverse side effects which are beyond the scope of this article.
Pitfall: If a patient is prescribed a higher tapering dose of steroids during a hospitalization ensure the maintenance dose of prednisone is continued after the taper.
Alternative Immunosuppressives
These are not commonly used, but may be required if there is a contraindication to one of the standard immunosuppressive agents.
Belatacept
Blocks costimulatory molecule (CTLA 4) that is required for activation of T cells.
- Can be given in lieu of a calcineurin inhibitor if there are concerns of adherence or calcineurin inhibitor contraindicated because of toxicity
- Given intravenously.
- Initial: Every 2 weeks for 5 doses while calcineurin inhibitor is being weaned
- Maintenance: Every 2 weeks
- Most often used concomitantly with an antimetabolite and glucocorticoid.
Adverse Effects
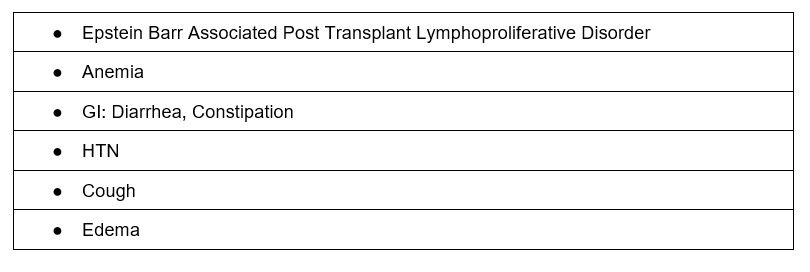
Mammalian Target of Rapamycin (mTOR) inhibitors
Impair DNA and protein synthesis thereby impairing proliferation of T and B lymphocytes as well as natural killer cells.
- Two mTOR inhibitors
- Sirolimus (more commonly used)
- Everolimus
- Trough levels monitored.
- Drug interactions similar to those for calcineurin inhibitors (see table above)
- Has antineoplastic properties.
- Can be used in lieu of calcineurin inhibitor along with antimetabolite +/- glucocorticoid if calcineurin inhibitor cannot be used.
- Can be used in lieu of antimetabolite if malignancy of other antimetabolite toxicity (i.e. leukopenia) precluding use.
- When used with a calcineurin inhibitor, typically tacrolimus is used. Sirolimus can increase cyclosporine levels and have cumulative adverse effects.
Adverse Effects

Summary
Immunosuppressant medications are the cornerstone of kidney transplant management. These medications have a number of important adverse effects and drug interactions to be aware of. It is important to know:
- Adverse effects
- Drug interactions
- Which medications can safely be held acutely