Table of Contents
What is the Anion Gap?
The anion gap is used in the diagnosis and evaluation of metabolic acidosis and is calculated by the following equation.
Na+ – (Cl– + HCO3–)
In reality all of the positive and negative charges in the body are equal. This equation only includes the major anions and cations. The anion gap is the difference between unmeasured anions (negatively charged particles) and unmeasured cations (positively charged particles).
Anion gap = unmeasured anions – unmeasured cations
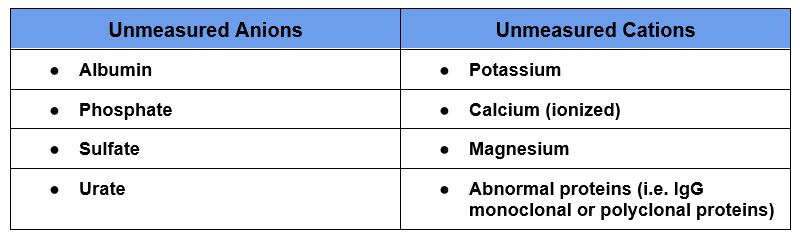
There typically are more unmeasured anions than unmeasured cations. For this reason there is an anion gap.
The main unmeasured anion is albumin. For this reason the anion gap can be corrected for hypoalbuminemia.
Corrected anion gap = Anion Gap + [2.5 x (4.5 – albumin)]
The normal anion gap may vary by laboratory as the sensitivity for measuring Cl– differs depending on the assay used.
I have seen normal ranges of:
- 3 – 9 mEq/L
- 8 – 12 mEq/L
- 6-14 mEq/L.
An anion gap of less than 3 mEq/L would universally be considered low.
Causes of a Low Anion Gap
The most common cause of a low anion gap is lab error. However a true low (or even negative) anion gap can occur in 4 situations
- Underestimation of serum sodium
- Overestimation of serum chloride
- Decreased unmeasured anions
- Increased unmeasured cations.
Underestimation of Serum Sodium
An artificially low reading of serum sodium can result in an abnormally low anion gap. This can occur in the following situations.
- Severe hypernatremia. The lab assay is less accurate and underestimates the true sodium in cases of severe hypernatremia (Na+ > 170 meq/L).
- Pseudohyponatremia occurs in cases of severe elevation in the solid phase of plasma proteins or lipids, typically severe hypertriglyceridemia.
Hyponatremia What the labs tell you. A deeper understanding of the physiology@BCNephro
Hyponatremia: What the labs tell us | BCNephro
Overestimation of Serum Chloride
An artificially high reading of serum chloride can result in an abnormally low anion gap. This is referred to as pseudo hyperchloremia. It occurs in the presence of exogenous anions that cross react with the Cl– lab assay. These are bromide and iodide.
- Bromide intoxication – Bromism. Bromide may be in certain medications and chronic use or overuse of these medications can result in Bromide intoxication. Bromide previously was in over-the- counter medications and sedatives, but was removed because of this complication.
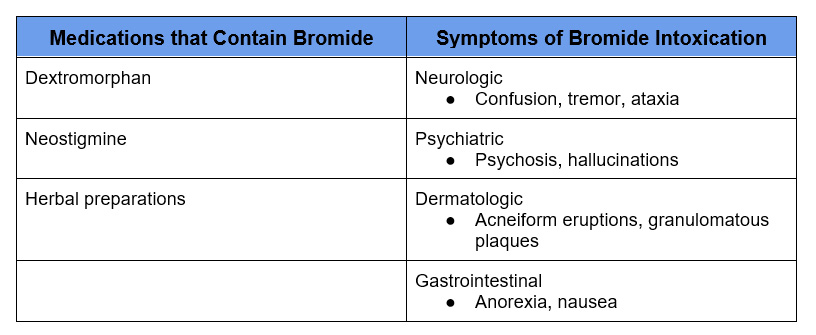
- Iodide – rare cause of pseudo hyperchloremia.
- Salicylates and thiocyanate may also cause pseudo hyperchloremia.
Decreased Unmeasured Anions
The most common cause of decreased unmeasured cations is hypoalbuminemia, which is the second most common cause of a low anion gap.
Corrected anion gap = Anion Gap + [2.5 x (4.5 – albumin)]
Albumin also acts as a buffer. Therefore severe hyperchloremic metabolic acidosis can decrease the net negative charge of albumin, decreasing the anion gap without hypoalbuminemia.
Increased Unmeasured Cations
Unmeasured cations refers to cations not in the equation Na+ – (Cl– + HCO3–), so cations other than Na+. Some of these are routinely measured on lab chemistries.
This can occur with:
- Hypercalcemia
- Hypermagnesemia
- Abnormal proteins: Typically paraproteins from either a monoclonal or polyclonal gammopathy. A finding of a low or negative anion gap should lead to an assessment for paraproteins
- Lithium: In a patient on lithium a low anion gap can be a clue to lithium toxicity. Typically occurs with lithium level > 4 meq/L
Summary
The finding of a low anion gap may be a clinical clue to other clinical conditions in which there may be an artificial lab interference of sodium or chloride, decreased unmeasured anions, or increased unmeasured cations.
For another review check out this article: