Table of Contents
Nephrologists generally use the physiologic approach to diagnosing acid base disorders. Check out this article for an overview of the physiologic approach.
Understanding Acid Base Disorders | BCNephro
However, there are two other approaches to diagnosis of acid base disorders.
- Base Excess
- Physicochemical (Stewart)
The Base Excess is routinely reported with blood gas results so we should know how to interpret it. This article will address the following:
- What is base excess?
- History of base excess
- How to use base excess
- What is the utility of the base excess?
For an excellent review check out this article:
Diagnostic Use of Base Excess in Acid–Base Disorders | NEJM
What is Base Excess?
Base Deficit is often referred to when determining if a patient has a metabolic acidosis. However the blood gas reports the Base Excess and what it really is reporting is the Standard Base Excess.
Base Excess is the amount of strong acid necessary to be added to one liter of blood to restore normal acid base balance (pH 7.40 and pCO2 40)
Standard Base Excess is a correction for the buffering capacity of Hemoglobin in vitro. Hemoglobin acts as a buffer, but in the body it is only in the intravascular space, not the entire extracellular pace. In the lab test, hemoglobin is present in the entire sample .
Base Deficit is the negative of the Base Excess.
I.e. a Base Excess of – 10 = a Base Deficit of 10
History of Base Excess
The concept of Base Excess was developed as a result of the polio epidemic.
- At that time the only acid base test available was the total blood CO2. (The total blood CO2 is a sum of the bicarbonate (HCO3–), dissolved CO2 and carbonic acid). This is what is measured on serum metabolic panel samples in the present day. Since the majority (@ 95%) of the total blood CO2 is HCO3– these are used interchangeably.
- Patients with bulbar polio were found to have an elevated total blood CO2. Initially they were thought to have a metabolic alkalosis of undetermined etiology. Subsequently, it was realized the total blood CO2 elevation was compensation for chronic respiratory alkalosis.
- Blood was analyzed with a pH meter. Using the pH and total blood CO2 (HCO3– ) the pCO2 was calculated using the Henderson Hasselbalch equation.
- These clinicians developed the Base Excess in an attempt to simplify assessment of the metabolic component of Acid Base disorders.
How to use Base Excess in Diagnoses of Acid Base Disorders
There are 3 steps.
- Determine the primary disturbance using pH, base excess, and pCO2
- Assess the secondary compensatory response
- Partition the standard base excess (or calculate the anion gap)
- If anion gap elevated (>15-20) calculate Δ – Δ and osmolar gap
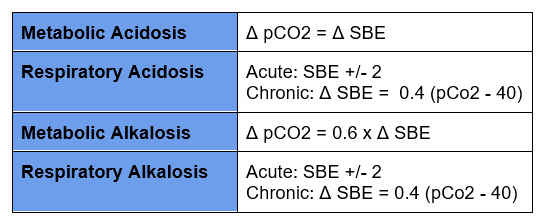
Determine the Primary Disturbance

Assess the Compensatory Response
Partition the standard base excess (or calculate the anion gap)
The next step is to assess for unmeasured anions. This can be done by partitioning the Standard Base Excess into that attributable to free water, chloride and albumin.
- SBE Free water = 0.3x (Na -140)
- SBE Cl– = 104 – (Cl– x 140/Na)
- SBE Albumin = (0.148 x pH-0.818) x (40 – Albumin grams/liter)
SBE unmeasured anions = SBE – SBEfw – SBEcl – SBEalb
Or you can assess the unmeasured anions by calculating the anion gap.
Anion gap = Na+ – (Cl– + HCO3–)
If the anion gap is elevated further evaluation can include:
- Calculate the Δ – Δ
- Calculate the osmolar gap
What is the Utility of the Base Excess
- The Base Excess approach is arguably simpler than the physiologic approach as the compensatory formulas may be easier to remember.
- The Base Excess provides prognostic information in certain settings, particularly trauma.
Summary
As a nephrologist, I prefer the physiologic approach to diagnosis of acid base disorders. However, the base excess is routinely reported on blood gas analysis and provides prognostic information in certain settings. This makes it important to understand how to use it.