Table of Contents
Overview
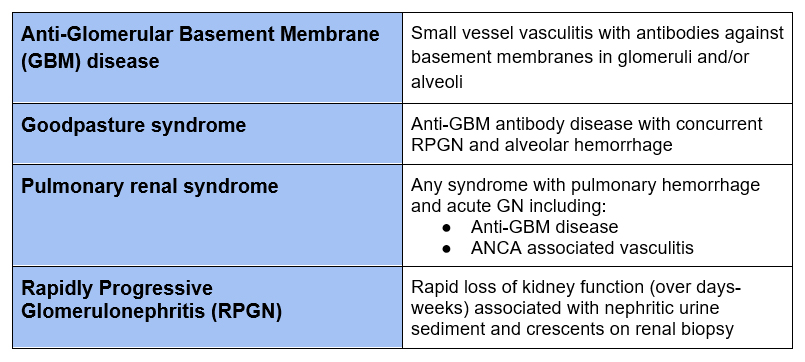
This describes a small vessel vasculitis with antibodies against the glomerular and/or alveolar basement membranes in capillaries.
- Glomerular Basement Membranes (GBM) and Alveolar Basement Membranes contain type 4 collagen with α 3,4,5 chains
- In this syndrome autoantibodies are formed against the non collagenous domain of the α3 chain of type IV collagen (α3(IV)NC1). Typically the antibody is IgG (IgG1 or IgG3)
- This manifests clinically as
- RPGN/ Crescentic glomerulonephritis: 80 – 90%
- Concurrent Alveolar hemorrhage: 40 – 60%
- Small minority isolated alveolar hemorrhage
Terminology
Demographics
- Rare condition (1.6 per 1 million population)
- More common with European and Asian ancestry. Rare with African ancestry
- Approximately 10-15% of RPGN will be anti GBM disease
- Can occur concomitant with ANCA associated, pauci-immune glomerulonephritis
- Bimodal age distribution
- 20’s – Pulmonary renal syndrome more common
- 50’s-60’s – Isolated renal involvement more common.
Pathogenesis
- Exposure to an environmental antigen (i.e. respiratory infection) in a patient with genetic susceptibility (HLA DR2, HLA DR 15) leads to autoantibody (GBM antibody) production.
- Damage to basement membranes exposes antigen (α3(IV)NC1) allowing it to be detected by the immune system
- Causes of Renal (Glomerular Basement Membrane) damage leading to susceptibility
- ANCA
- Membranous Nephropathy
- Lithotripsy
- Causes of Pulmonary (Alveolar Basement Membrane) damage leading to susceptibility
- Smoking
- Exposure to hydrocarbons (fumes from gasoline, kerosene, paint thinner etc)
- Alemtuzumab (monoclonal anti CD52 antibody) via loss of regulatory T cells
- Causes of Renal (Glomerular Basement Membrane) damage leading to susceptibility
Clinical Presentation
- Acute onset
- Often prodromal respiratory infection
- Rapidly Progressive Glomerulonephritis (RPGN). Creatinine worsens over days – weeks. Nephritic urine sediment with glomerular hematuria and proteinuria.
Atypical Anti-GBM antibody disease
- In 10% Anti-GBM antibody not detectable
- Linear IgG staining on immunofluorescence of kidney biopsy
- Less severe clinical presentation (less severe kidney injury, biopsy with proliferative changes, fewer glomeruli affected with crescents, associated alveolar hemorrhage rare)
Diagnosis
Serologies
- Anti-Glomerular basement membrane antibody. Typically a send out test
- ANCA antibodies. There is a high incidence of ANCA positivity (usually MPO) in anti-GBM antibody disease. ANCA positivity affects prognosis and risk of relapse.
- 30-50% of patients with anti-GBM ab positivity will also be ANCA positive
- 10% of patients with ANCA positivity will also be anti-GBM antibody positive.
Renal biopsy
- Crescentic GN
- Crescents in 95%
- 80% have crescents in > 50% glomeruli
- Average number of glomeruli with crescents 75%
- Typically little chronicity (interstitial fibrosis, tubular atrophy, fibrous crescents)
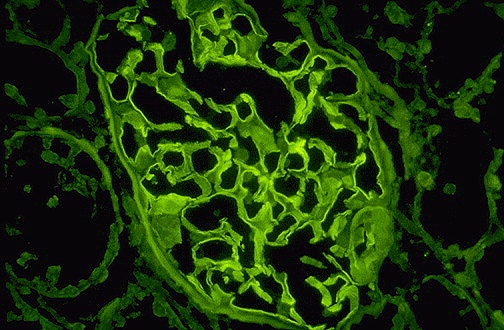
- Immunofluorescence
- Linear (ribbon like) staining for IgG
- Differential (pseudolinear)
- Diabetes
- Paraproteinemia
- Lupus Nephritis
- Fibrillary GN
- Electron Microscopy
- Typically no deposits
- Exclude concomitant membranous nephropathy or fibrillary glomerulonephritis
Treatment
Treatment of Anti-GBM antibody disease has 3 components
- Glucocorticoids
- Plasmapheresis
- Cytotoxic Therapy
It is important to diagnose and treat early. If patients have dialysis dependent kidney failure and 100% of glomeruli have crescents on biopsy renal recovery is rare.
Glucocorticoids
If there is a high index of suspicion initiate prior to confirmation of diagnosis with anti-GBM antibody and renal biopsy.
- Pulse methylprednisolone followed by
- Oral prednisone (60 mg/ day). Taper to 20 mg by 6 weeks, complete taper of total 6-9 months.
Plasmapheresis
- Daily plasmapheresis: Guidelines recommend 4 liter exchanges with albumin replacement. (I typically do 1.5 plasma volume exchanges)
- Add FFP: Guidelines recommend 300-600 ml (I typically do 25% or 2-4 units) if recent kidney biopsy (within 3 days) or other bleeding (such as pulmonary hemorrhage)
- Course of plasmapheresis typically 2-3 weeks (continue until anti-GBM antibody becomes undetectable)
Cytotoxic therapy
- Oral cyclophosphamide. (More efficacious than IV pulse cyclophosphamide)
- 2-3 mg/kg per day for 2-3 month
- Cyclophosphamide renally excreted.
- Use attenuated (i.e 50%) dose if decreased eGFR
- 2 mg/kg per day if age > 55
- Follow CBC with differential at least twice monthly while on cyclophosphamide given risk of leukopenia. Hold if WBC < 4 and resume at attenuated dose when WBC > 4.
Novel treatment
- Imlifidase: An IgG degrading enzyme produced by strep pyogenes
- I have not seen this drug used.
TIPS
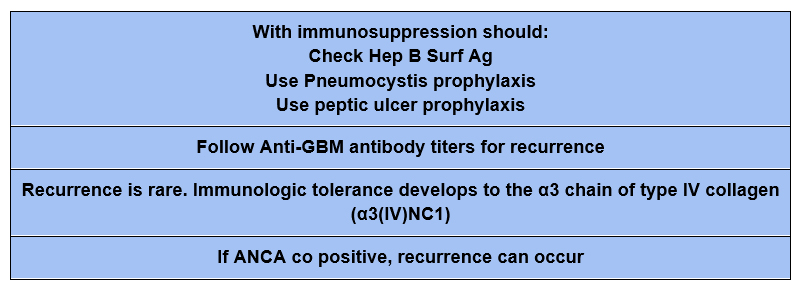
Prognosis
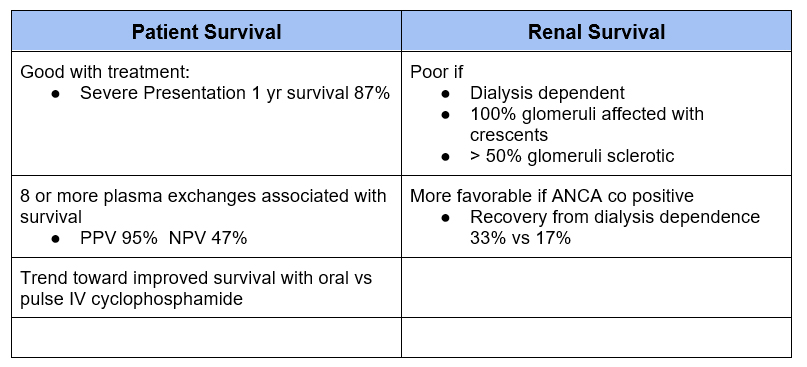
If the patient is dialysis dependent and has 100% glomerular involvement with crescents the chance of renal recovery is so low that some recommend foregoing treatment given the risks of immunosuppression.
I personally would favor initial treatment (at least until anti GBM antibody is negative) to mitigate the possibility of developing pulmonary hemorrhage.
Kidney Transplant
- Kidney transplant should not occur unless anti-GBM antibodies are negative (for 6 months).
- Recurrence after kidney transplant is rare. (2.7%)
- Denovo anti-GBM antibody disease can occur after transplant for Alport’s syndrome.
- Alport’s syndrome is a defect in alpha 3 or alpha 4 (in AD or AR) or alpha 5 chain (X-Linked) of type 4 collagen. Therefore the transplant kidney has the normal antigens in type 4 collagen that the patient’s immune system may not have been exposed to because of the genetic defect.
- 5-10% of these patients will develop anti-GBM antibodies, although clinical GN is rare.
Summary
Anti GBM disease is a rare small vessel vasculitis that can result in RPGN and pulmonary hemorrhage. Although it is rare, it comprises about 10-15% of cases of RPGN. There often is concurrent ANCA positivity. Treatment is effective, although if diagnosis and treatment is delayed until dialysis dependent and 100% of glomeruli affected by crescents renal recovery is rare.