Table of Contents
A normal sodium on a lab test is 140 mEq (or mmol) / liter, but normal saline (which is purportedly isotonic to plasma) is 154 mEq/liter. What gives?
This article will describe how sodium is measured clinically and explain this apparent discrepancy.
How Sodium is Measured
Historically sodium was measured by flame photometry, current technology uses ion selective electrodes.
Flame Photometry
How does flame photometry work?
When electrolytes are excited they emit different color light.
- The specimen (serum or plasma) is diluted.
- When exposed to the flame the sodium atoms become excited and emit a certain color.
- The intensity of the color is measured.
- The sodium concentration is determined based on this intensity.
This process is time consuming and labor intensive.
Ion Selective Electrode
Currently ion selective electrodes are used to measure electrolytes. This process is often automated.
- The specimen can either be non-diluted (direct) or diluted (indirect)
- Direct (non-diluted) specimens of whole blood are typically used in blood gas analyzers
- Indirect (diluted) specimens of serum or plasma are typically used in the cynical chemistry lab.
- It is exposed to an ion selective membrane (permeable only to sodium)
- The electrical potential difference is measured and reported in mEq/Liter
What is the Difference Between Milliequivalents and Milliosmoles?
A millimole (mmol) is a quantity. 1/1000 of a mole.
A milliequivalent (mEq) is the electrical charge of an ion. 1/1000 of an equivalent.
A mEq is the chemical activity of a substance relative to 1 mg of hydrogen (H+). For monovalent ions (Na+, K+, Cl–) mEq and mmol are equal. For ions that are not monovalent (Ca++, Mg++), mEq are mmol x the ion charge (valence). This is 2x for Ca++ and Mg++. Calcium and magnesium are often reported in mg/dL, therefore conversion to mmol/L also involves the molecular weight.
What is the Difference Between Plasma and Serum?
Sodium can be measured on:
- Whole blood
- Serum
- Plasma
Whole blood is used when electrolytes are measured by blood gas analyzers.
The clinical chemistry lab typically uses serum or plasma specimens.
What is in Blood?
Although blood is typically considered a liquid, it contains both liquid and solid substances including:
- Cells. Mainly red blood cells, but also white blood cells and platelets.
- Solid phase (i.e. plasma proteins and lipids).
- Aqueous phase (liquid with dissolved electrolytes).
In a blood specimen the volume of red blood cells is the hematocrit. The remaining (serum or plasma) is mainly liquid (aqueous phase) with solid things (lipids and proteins) floating around in it.
Typical serum or plasma is comprised of 93% liquid and 7% solid
Serum and plasma both come from the liquid portion of blood, what remains after RBCs are removed.
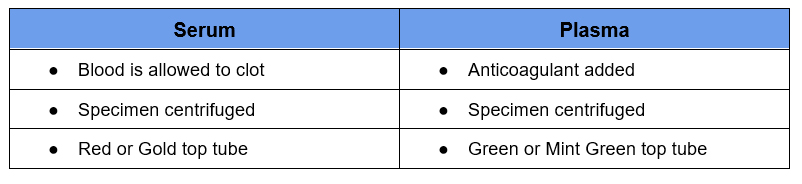
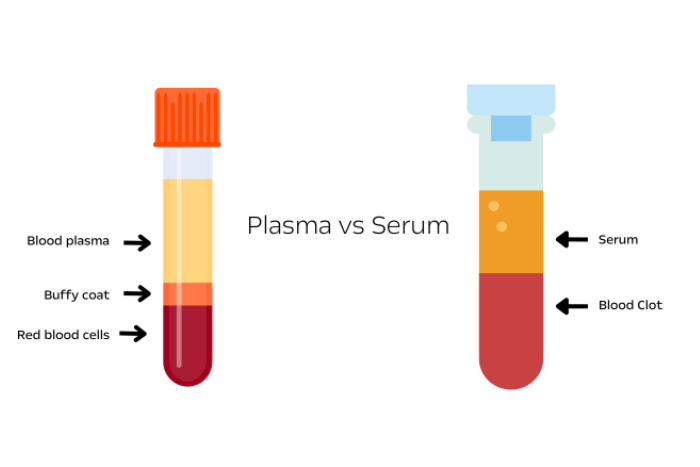
The type of specimen affects potassium as some potassium leaks out of cells during the clotting process. Therefore the normal range of potassium is higher for a serum vs a plasma specimen. It does not have a significant effect on sodium.
Normal Sodium
A normal sodium concentration depends on what volume is considered.
Sodium concentration in plasma = 140 mEq/L
However, the majority of sodium is in the liquid aqueous phase of plasma.
Sodium concentration in the aqueous phase of plasma = 140/0.93 = 150.5 mEq/L
Pseudohyponatremia
Pseudo (or false) hyponatremia is when the lab calculates a low sodium, but the actual sodium is normal. This can occur when the specimen is diluted or indirect as in flame photometry and most automated clinical ion selective electrode lab measurements.
Because the specimen is diluted, the number measure is not 140 mEq/Liter. A calculation is used to convert the measured number to what the expected undiluted value would be. This uses the assumption that the serum or plasma specimen is normal, 93% aqueous and 7% solid.
There are certain conditions with increased plasma proteins or lipids where the solid phase is greater than 7% as in severe hypertriglyceridemia. In these situations the calculation is not valid and will result in a falsely low sodium reading.
Summary
Electrolytes such as sodium are dissolved in the liquid or aqueous phase of plasma. The concentration depends on whether the total volume of serum or plasma (including solid proteins and lipids) or just the aqueous volume is considered. Conventionally clinical laboratories consider the total volume, whereas the concentration of intravenous normal saline was developed based on the concentration of the aqueous phase. Dilution (indirect) analysis requires a conversion calculation which can result in pseudohyponatremia if there is an increase in plasma proteins or lipids.